In support of World Cervical Cancer Elimination Day
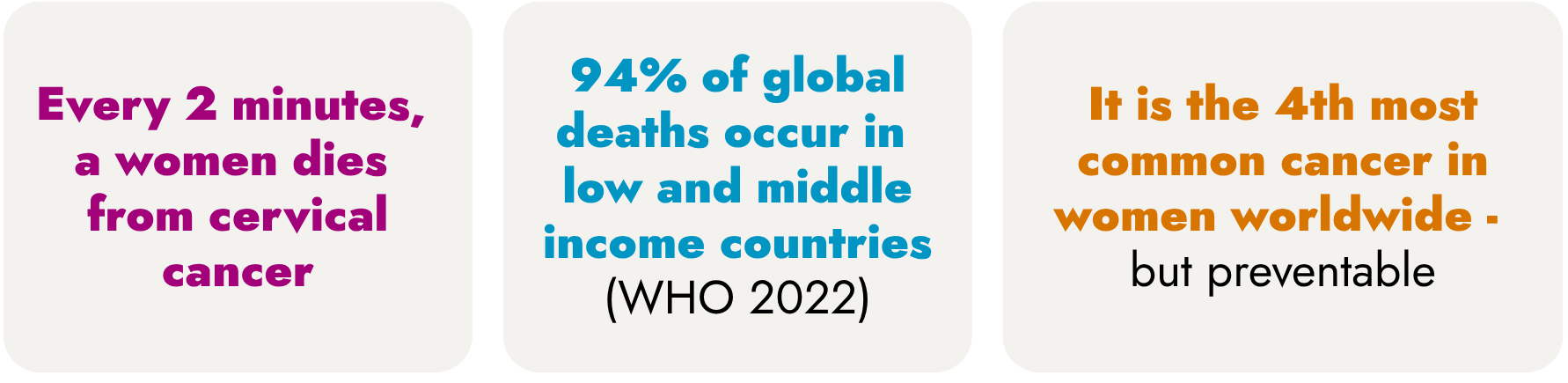
World Cervical Cancer Elimination Day began on 17 November 2020, when 194 countries collectively committed to eliminate a cancer for the first time. Sadly, the statistics are still alarming:
This year’s theme: “Act Now: Eliminate Cervical Cancer” calls for bold, united action to build on existing progress and accelerate impact toward the World Health Organization’s 90-70-90 targets by 2030.
TruScreen’s innovative, real-time, AI-enabled technology was designed to make cervical screening more accessible, accurate, and sustainable - to support increased screening rates globally - in particular, WHO member nations hoping to achieve the target of 70% of women screened with a high-performance test by age 35 and again at 45, by 2030.
TruScreen represents a next-generation alternative to traditional cytology and biomarker-based assays, offering an instant, real-time assessment of cervical tissue through advanced optical and electrical analysis of cellular and subcellular changes.
By eliminating the need for laboratory infrastructure, consumables, and slide interpretation, TruScreen provides an efficient, objective, and real-time triage solution, particularly valuable in low-resource settings facing significant barriers such laboratory dependence, high costs, infrastructure requirements, and loss to follow-up. 169 of 194 WHO member nations are emerging economies facing such challenges.
The TruScreen device has now been clinically validated by over 30 published trials to date, with large clinical studies including over 40,000 women in multiple settings. TruScreen has improved performance comparable to liquid-based cytology for detecting CIN2+ lesions while eliminating subjectivity, sample adequacy issues, and processing delays. Unlike cytology, TruScreen examines deeper tissue layers beyond surface epithelial cells, potentially providing more comprehensive tissue assessment. The elimination of failed samples represents a significant operational advantage.
“TruScreen represents a reliable, practical screening tool for cervical neoplasms and provides an evidence-based approach for policymakers when selecting the optimal cervical cancer screening strategy in countries without an established national screening program.”
- Majed Alhudhud, Shazia Maqsood, Maab El Hussein et al. Beyond Tradition: Investigating TruScreen's Performance Versus Pap Smear in Cervical Cancer Detection, 25 July 2024
TruScreen has now screened over 1 million women to date in over 20 countries. We’ve trained over 1000 healthcare professionals in 231 hospitals or clinics worldwide, and currently have 247 devices operational globally.
TruScreen has been selected for use in three Public Screening Programs in 2026 (so far):
Vietnam - 260,000 women in Ho Chi Minh City, with planned national expansion in a country with an estimated 35m women of screening age
Zimbabwe - 20,000 women in Masvingo province with planned expansion
Uzbekistan - 500 patient pilot project in Karakalpakstan with planned expansion
TruScreen has achieved multiple international regulatory approvals, including the CE Mark, TGA, and NMPA (China) certifications, and is currently registered and approved for cervical cancer screening in 14 countries. Its clinical relevance has been recently cited in the UNITAID’s 2024 Screening and treatment of precancerous lesions for secondary prevention of cervical cancer Technology landscape report and in several national and professional guidelines (China, Russia, Vietnam and Mexico, among others).
How it works
TruScreen directly assesses the cervical tissue for precancerous and cancerous changes, and provides an instant result during the patient visit, enabling immediate counselling and clinical action. It does not rely on laboratory samples such as Pap smears or HPV tests and ensures equitable access to early detection.
Key Advantages
Real Time Results: Screening and reporting completed in one visit.
Non-Invasive: Utilizes optical and electrical tissue evaluation without any scraping of the cervix to collect tissue samples.
Ideal for Low-Resource Settings: Requires no laboratory infrastructure or cold chain logistics.
Proven Performance: Clinically validated in multiple countries, with WHO-aligned sensitivity for detecting high-grade lesions.
Sustainable Model: Low operational costs and minimal waste generation.
Work with us to eliminate cervical cancer
We would be delighted to explore how TruScreen could enhance the impact of your initiatives to help eliminate cervical cancer.