Eliminating Cervical Cancer
Our vision is a world without cervical cancer.
In late 2020, our vision was validated by the first-ever global commitment to eliminate a cancer – cervical cancer.
The World Health Organisation’s (WHO) Global Strategy to Accelerate the Elimination of Cervical Cancer was launched in November 2020, outlining a key target of achieving 70% screening rate using a high performance test by the age of 35 (and again by the age of 45), with the goal of reducing over 40% of new cervical cancer cases and over 5 million deaths in the next 30 years.
-
Cervical cancer is the fourth most common cancer in women globally with around 660 000 new cases and around 350 000 deaths in 2022 (WHO).
-
Every 2 minutes, a women dies from cervical cancer.
-
Without comprehensive control measures, cervical cancer incidence and mortality rates are expected to worsen.
-
Cervical cancer is a preventable disease - in fact it is only one of a handful of cancers with a known cause.
-
94% of global deaths from cervical cancer occurred in low and middle income countries in 2022 (WHO).
169 of 194 WHO member nations are emerging economies with limited or no screening laboratory infrastructure - economies which TruScreen’s screening technology is well suited to support.
TruScreen already has current sales or market entry activity in 36 WHO member nations, with an addressable market of 1.3 billion* women of screening age.
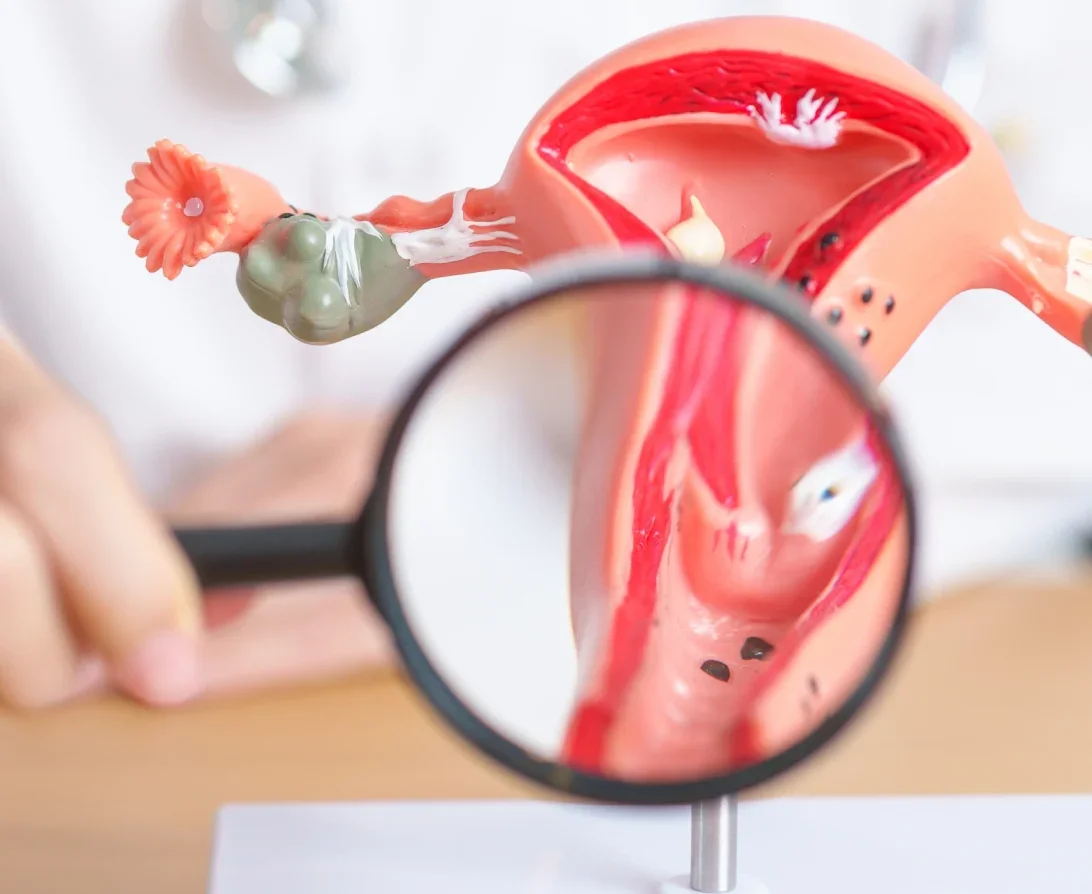
What is cervical cancer?
Cervical cancer is the growth of abnormal cells in the lining of the cervix.
Cervical cancer develops slowly and is strongly linked to human papillomavirus (HPV) infection, HPV is found in 99% of cervical cancer cases. Pre-cancerous cervical cells may not cause symptoms and by the time symptoms become apparent, it is often too late for treatment – particularly in resource-poor environments.
Progression of HPV infection to cancer is slow, generally taking more than 10 years. Although invasive cancer is most commonly seen in women aged over 45 years, the precursor changes are detectable much earlier through routine screening.
If early changes in cervical cells are detected through screening programmes, cervical cancer is curable. However, if undetected until late in its clinical course, it has a high death rate.
Some countries with effective screening programmes have reported significant reductions in the burden of cervical cancer⁵.
Cervical cancer screening options
Organised screening programmes involve the routine testing of an asymptomatic “healthy” population. Screening can reduce rates of both cervical cancer incidence and mortality by detecting precancerous lesions (hence preventing cancer) and detecting invasive cervical cancers at an early stage, thereby increasing patient survival.
There are numerous primary cervical cancer screening methods.
-
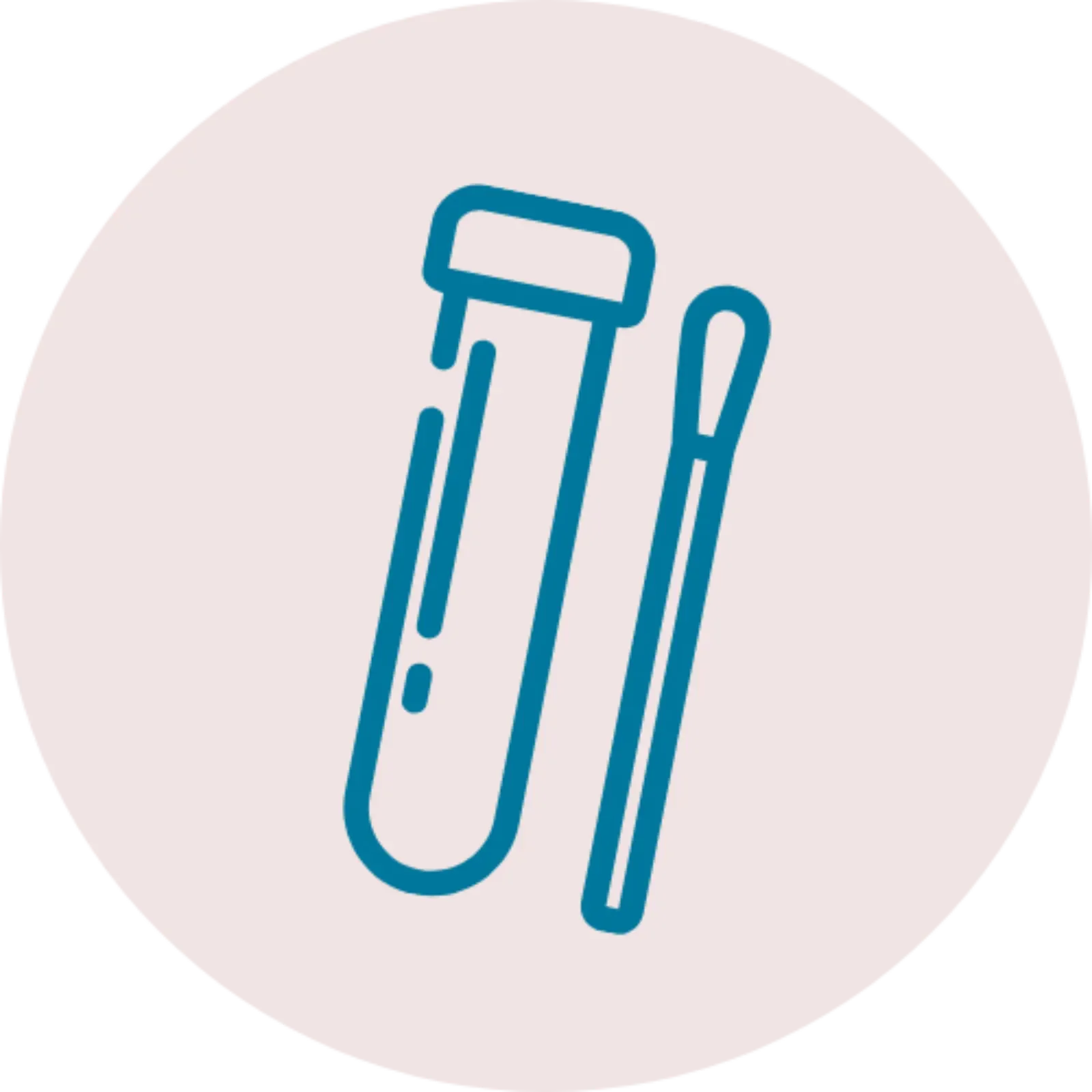
Pap test
A Pap Test, also known as a Pap Smear, is a conventional cytology method of screening that looks for cell changes in the cervix. A Pap Test involves a doctor scraping a sample of cells from a patient’s cervix. The sample is then sent to a laboratory to be tested for the presence of abnormal cells.
Invented in the 1920’s Pap Tests have long been considered the golden standard in cervical screening and were a significant breakthrough in women’s health. Although critical in the creation of cervical screening programmes, Pap Tests are starting to be phased out and replaced with newer methods.
-

Liquid Based Cytology (LBC)
Liquid Based Cytology (LBC) is a newer variation of the conventional Pap test. The cervical cells collected at the point of screening are suspended in liquid. This liquid sample is then sent to a laboratory where it is flited to remove unnecessary material and examined under a microscope.
-

HPV DNA Testing
DNA Testing for HPV is a more modern screening method. A patient’s cells are examined in a laboratory for the genetic material of specific high-risk oncogenic HPV strains. If evidence of HPV is identified, HPV genotyping will be performed to determine the specific strain causing the infection.
HPV DNA screening is most often accompanied by triage testing as the presence of HPV does not necessarily mean the patient has any pre-cancerous or cancerous changes on her cervix, only that she is potentially at risk.
If a patient is found to have a high-risk HPV strain, she will often need to then undergo further screening tests to determine the next steps and minimise overdiagnosis. Australia’s National Cervical Screening Programme uses LBC to triage HPV-positive women, which increases the cost of screening but can significantly reduce over treatment by minimising referrals to colposcopy when compared to HPV DNA testing alone.
Although HPV DNA testing has started to dominate modern screening guidelines in high income countries, it is a quite expensive process that will not be affordable for population based screening in many LMICs.
-

Visual Inspection
Visual Inspection with Acetic Acid (VIA or VIAC) are commonly used in remote or low-resource settings.
VIA begins with the cervix being swapped with a solution of dilute acetic acid. The solution interacts with the cells on the surface of the cervix, this reaction is then visually inspected. In VIAC once the visual inspection is complete a camera with a special lens is used to photograph the cervix to help identify the presence of abnormal cells.
VIA and VIAC are relatively inexpensive methods and provide immediate results. However, they are relatively non-specific and have low sensitivity. VIACs are extremely reliant on the training of the clinician performing the exam.
The TruScreen Method
TruScreen resolves many of the ongoing issues with conventional screening methods - substantial infrastructure, highly trained human resources, and robust quality assurances − most of which are not widely available in LMIC’s or other low-resource settings.
TruScreen provides an alternative primary cervical screening solution that addresses these barriers. The TruScreen device offers the latest technology in cervical cancer screening, providing real-time detection of pre-cancerous and cancerous cervical cells to help improve the health and well-being of women around the world.
-
Immediate feedback to patient and operator. Patient can be treated if necessary at time of visit. Patient not lost to follow-up with delayed reporting.
-
Reproducible, consistent results to confirm accuracy.
-
Greater access to women in remote communities. Easy to use. No qualified cytologists needed. Suitable for remote areas and developing countries. Cost savings in resources / overheads.
-
Assured level of performance.
High standard of cervical screening. Improved ability to detect disease and save lives.
Economic savings to global healthcare systems.
Low and Middle Income Countries (LMICs) need an innovative solution like TruScreen to achieve screening goals
The World Health Organization’s global strategy is designed to advance women’s health, strengthen global health systems and put countries on the right path to eliminating cervical cancer. This is the first time the world has had the evidence, capabilities and cooperation to enable the elimination of a major cancer.
In addition the elimination strategy, WHO updated its cervical cancer screening and treatment guidelines in 2021, highlighting a +5.5% increase on estimated diagnoses (2018-2020) and a +9.9% growth in the estimated numbers of deaths (2018-2020)
Most importantly, the updated guidelines clearly state that conventional screening methods have not been successful in LMICs. These regions will need to adopt innovative screening methods if they are to meet the global WHO targets.
In 2024, Unitaid published their review of the the cervical cancer screening technology landscape - and brought TruScreen’s AI-based opto-electrical device to the attention of the global cancer screening community.
Let’s work together
If you would like to discuss how TruScreen can support your market, we’d love to hear from you.
Can’t see the form? Email us at info@truscreen.com.