A world without cervical cancer.
Enabled by AI, TruScreen provides an accurate, real-time cervical cancer screening solution.
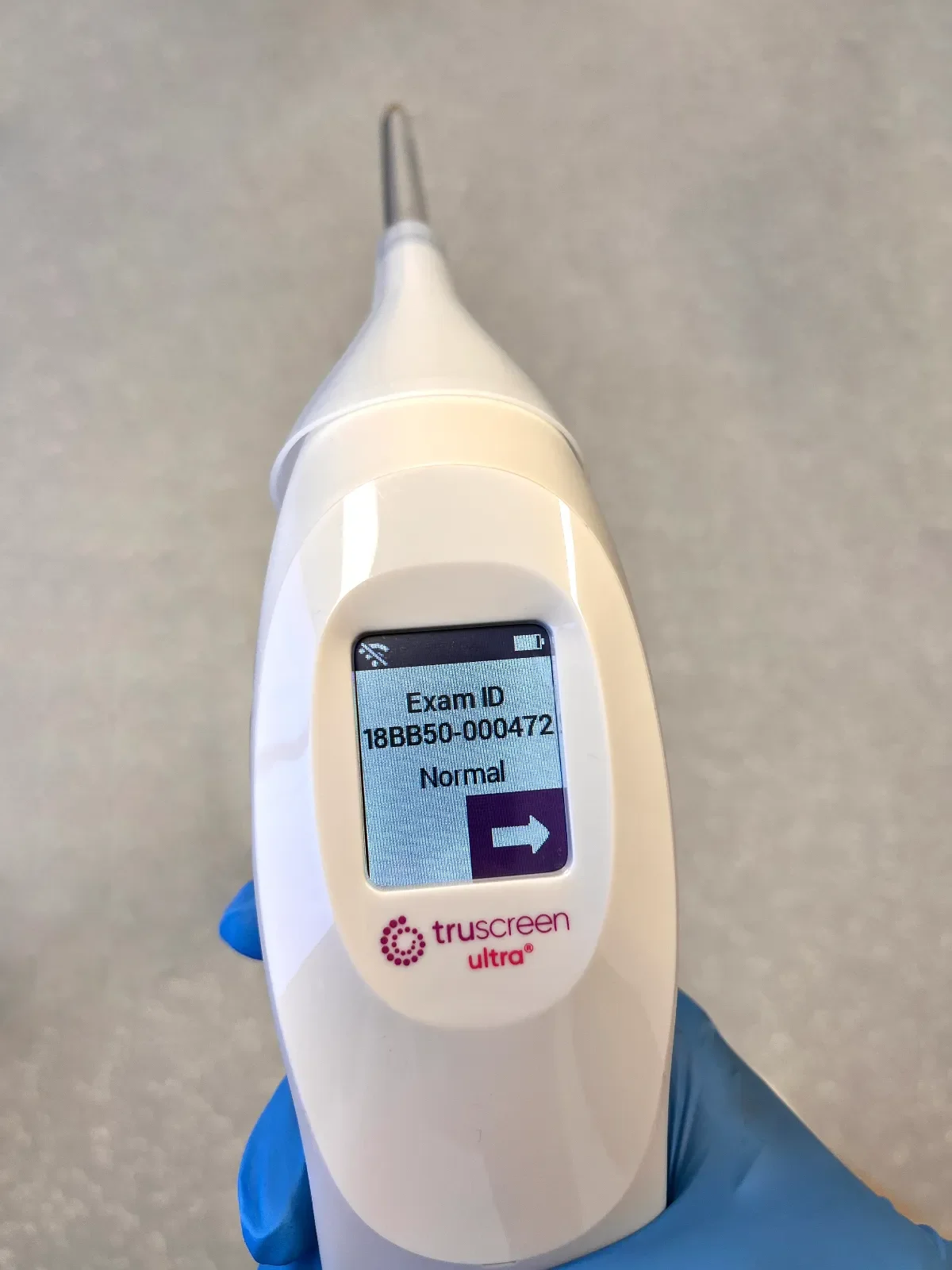
Over 1,000,000 women have been screened with TruScreen to date, with a robust safety profile.
Our market potential is huge with 1.256 billion women eligible for screening in our key markets
Demonstrating a continued commitment to cervical cancer screening solutions.
Globally, an estimated 662,044 cases from cervical cancer occurred in 2022 - the fourth biggest cause of cancer morbidity and mortality in women.
-
Immediate feedback to patient and operator. Patient can be treated if necessary at time of visit. Patient not lost to follow-up with delayed reporting.
-
Reproducible, consistent results to confirm accuracy.
-
Greater access to women in remote communities. Easy to use. No qualified cytologists needed. Suitable for remote areas and developing countries. Cost savings in resources / overheads.
-
Assured level of performance.
High standard of cervical screening. Improved ability to detect disease and save lives.
Economic savings to global healthcare systems.
Investing in cervical cancer screening is highly impactful
-

High Burden
Cervical cancer is one of the most common cancers among women, especially in low to middle-income countries where screening rates are low.
-

Preventable
With effective screening and early detection, cervical cancer is highly preventable. Technologies like TruScreen can significantly reduce incidence and mortality rates.
-

Cost effective
Early detection and treatment are far less expensive than treating advanced cancer, making it a cost-effective investment for healthcare systems.
-

Market Potential
There’s a growing demand for innovative, accessible, and affordable healthcare solutions in emerging markets.
-

Social impact
Improving women’s health has a ripple effect on families and communities, enhancing overall societal well-being.
Current and upcoming public screening programs:
Vietnam
A 5-year program to screen 260,000 women for cervical cancer using TruScreen in Ho Chi Minh City launched in July 2025. The program is a partnership between TruScreen, Ho Chi Minh City Public Health Association and distributor Gorton Health Services, and will assist goal of screening 60% of women aged 30 to 54 for cervical cancer (currently 25%).
Zimbabwe
Since 2019, TruScreen has screened 33,000 women in the Masvingo Province, Zimbabwe, through a public screening program managed by the Zimbabwe National AIDS Council (NAC) and the Ministry of Health and Childcare. Re-validation has been completed in order to recommence and expand the program to the Harare and other provinces - with 20,000 women expected to be screened in 2026.
Uzbekistan
Following TruScreen’s receipt of regulatory approval by the National Pharmaceutical Safety Committee in Uzbekistan in June 2025, a 500 patient pilot project is planned to commence in FY2026 in Karakalpakstan, Uzbekistan. Uzbekistan has over 11 million women of screening age*, and is also a major healthcare reference site for neighbouring Central Asian nations.
*CIA World Factbook women aged 15-64
Key markets
TruScreen currently distributes to 19 countries, including three of the world’s four most populous nations - China, India and Indonesia.
Major International Approvals
CE Mark
TruScreen’s key quality certification is the CE mark (EU approval) and TruScreen is transitioning to the new European Medical Device Regulation (MDR).
National Medical Products Administration, China
In our largest commercial market, China, medical device regulation is regulated by the NMPA.
ISO 13485 and IEC 60601-1-2 Certified
TruScreen is committed to quality, safety, and regulatory compliance in the design, development, production, and service of it’s device.
Global Recognition
World Health Organisation (WHO)
UNITAID
Clinton Health Access Initiative
Daffodil Centre - Australia
China Obstetricians and Gynaecologists Association
China Society for Colposcopy and Cervical Pathology
Russia Cervical Cancer Screening Guidelines
Vietnam Ministry of Health Technical List
Regulatory approvals
Australia
Russia
Vietnam
Rwanda
UK
Mexico
Uzbekistan
Jordan
Saudi Arabia
Singapore
Indonesia
Zimbabwe
India
New Zealand
China
Sign up for regular
updates and
announcements.
Can’t see the form?
Email us at marketing@truscreen.com to subscribe